

There is a universal gas constant which relates these variables and the molecular weight of any gas. The density (specific volume), pressure, and temperature of a gas are related to each other through the equation of state. Either variable can be used to define the state of the gas, since they are reciprocals. When a gas is moving, it is more convenient to use the density (r) of a gas, which is the mass divided by the volume the gas occupies.
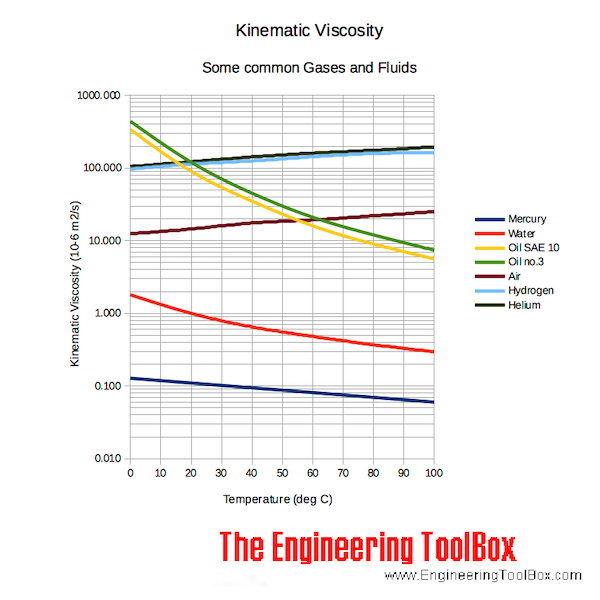
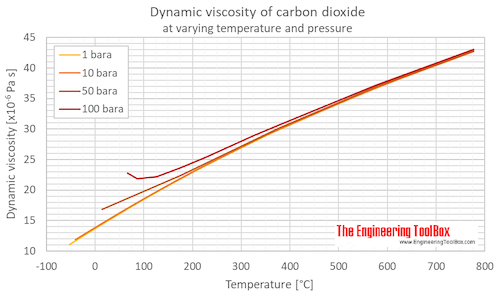
When working with a static (unmoving) gas, it is convenient to use specific volume (v), which is the volume divided by the mass. Since the mass and volume are directly related, we can express both the mass and volume by a single variable. For a given pressure and temperature, the volume depends directly on the amount of gas. The sum of the mass of all the molecules is equal to the mass of the gas.Ī gas occupies some volume in three dimensional space. The temperature (T) of a gas is a measure of the kinetic energy of the gas. A gas is composed^M of a large number of molecules which are in constant motion. This “sticky” property of the gas is called the viscosity (mu) and it plays a large role in aerodynamic drag. A gas can also exert a tangential (shearing) force on a surface, which acts like friction between solid surfaces. The pressure (p) of a gas equals the perpendicular (normal) force exerted by the gas divided by the surface area on which the force is exerted. The values and relations of the properties define the state of the gas. We usually model air as a uniform (no variation or fluctuation) gas with properties that are averaged from all the individual components.Īny gas has certain properties that we can detect with our senses. Home > Beginners Guide to Aeronautics Properties of Air – Text VersionĪir is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various other components.


 0 kommentar(er)
0 kommentar(er)
